Research Article - (2022) Volume 9, Issue 5
Received: Mar 08, 2022, Manuscript No. JEBMH-21-46330; Editor assigned: Mar 11, 2022, Pre QC No. JEBMH-21-46330(PQ); Reviewed: Mar 25, 2022, QC No. JEBMH-21-46330; Revised: Mar 30, 2022, Manuscript No. JEBMH-21-46330(R); Published: Apr 05, 2022, DOI: 10.18410/jebmh/2022/9.6.17
Citation: Poli V, Aparna Y, Renuka M, et al. Protective Effect of Vitamin C and E on Enzymatic and Antioxidant System in Liver and Kidney Toxicity of Cadmium in Rats. J Evid Based Med Healthc 2022;9(06):01.
Backgroud: The present study aimed to evaluate Heavy metals are persistent and uninterrupted ecological pollutants that are capable to cause numerous dysfunctions in target tissues of exposed animals as well as humans. Metallic components including Cadmium (Cd) arrive into the animal body then become stored mainly in the liver, kidney etc.
Methods: Despite the study of several years, no reports are available about actual medication for prolonged toxicity of heavy metals including Cd. Cd is one of the most common heavy metals that possess toxicological effects of numerous tissues of animal systems.
Result: In the present study an attempt was made to monitor the impact of Cd - toxicity in the tissues of wistar rats. BMR and TR potentials were found to be significantly (P < 0.05) inhibited, Tissue level oxidative metabolism represented by enzymes of Glycolytic and Kreb’s cycle enzymes were found to be significantly (P < 0.05) altered. Enzymes of glycolysis i.e. Phosphorylase ‘a’ and Aldolase were found to significantly elevated, whereas Kreb’s cycle and oxidative enzymes including SDH and Cytochrome-c-oxidase were found to be significantly inhibited, suggesting the metabolic shift from aerobiosis to anaerobiosis, visualized through increased lactate production at cellular level. Similarly antioxidant enzymes were also affected during Cd - toxicity, and stimulates the production of ROS, diminishing their structures, prohibition of the roles of antioxidants.
Conclusion: The findings from this study showed that a combination of Vitamin C and E enhances the biological recovery induced by Cd, pretreatment with antioxidants including Vitamin C and E decreases the oxidative stress, inhibits progressive fluctuations convinced through Cd, repaired the biochemical alterations and reverted to almost normal metabolic regimes. The addition of Vitamin C and E, to treat the detrimental effects caused due to Cd - toxicity, causing beneficial impacts including prevention and alleviation of Cd - toxicity. The individuals, who have high possibility of introduction to toxic metals should abundantly consume large quantities of fruits and vegetables on a regular basis, which are enriched with essential elements and vitamins. These nutritive complements have no side effects than chelation treatment as well as cost effective for individuals, who have unintentionally exposed to contamination of metal environs. So, nutrient supplements, which are enriched with natural antioxidants should be provided to overcome xenobiotic toxic stress.
Antioxidant enzyme, Vitamin C and E, Cadmium toxicity, Liver, Kidney
Environmental toxicants have become a major source of health hazards to humans thereby impacting negatively on health and overall well - being of exposed individuals. In recent years, industrial activities have been increased which leads to prolonged human exposure to industrial pollutant such as Cadmium (Cd) which is used in batteries, pigments electroplating, refinery, plastic and petrochemical industries and also arises from cigarette smoke.1,2 Many different forms of exposure to Cd have been shown over the past century, with Cd being present in the environment of many human activities. Cd is a non - biodegradable heavy metal which possess a relatively long (half - life) and readily accumulates in bodily tissues where in it produces tissue toxicities and subsequently leading to tissue dysfunction. Cd displays various mechanisms of toxicity in certain species under experimental conditions. Cd is a universal toxic metal that causes free radical production and stimulates Reactive Oxygen Species (ROS) such as hydrogen peroxide and thiobarbituric acid reactive substances that act to impair the anti - oxidant defense system.3,4
Highlighting a link between the occurrence of diseases and diet is an area of study that attracts the attention of many researchers. Particularly, the usefulness of certain recognized antioxidants including vitamins with relatively large antioxidant potential activity, to fight against free radicals and oxidative stress. In fact, because of the great reactivity and deleterious action of free radicals on biological systems, they are incriminated in mechanisms of ageing and also several pathophysiological conditions in the biological system.5 Strengthening the body’s antioxidant defense mechanisms, seems to be a major issue to combat oxidative stress and preserve health. Usually antioxidants may prevent the damage caused by free radicals to the body cells, prevent the body condition, thereby promoting longevity and warding off several diseases and disease conditions. Several natural antioxidants have been introduced to protect the cells, including Vitamin C (Ascorbic acid), Vitamin E (α - tocopherol) appear to be among the most commonly used antioxidants. This particular field of study in using natural antioxidants was booming and considerable data on the potential benefits of antioxidant supplementation have been reported in recent times.5,6,7
The use of antioxidants and their effects in the biological system were not fully understood. It has been shown that antioxidants may exhibit pro-oxidants activity depending on the dose and the oxidant / antioxidant status of the body.8 Therefore, the objective of this study was to examine the beneficial role of antioxidant vitamins in stress oxidative prevention, but also their potentially detrimental effects of Cadmium-induced damage by vitamin C and E supplementation.
Animals
Male rats weighing 225 ± 10 g were selected in the present study and were housed in stainless steel mesh cages, under standard laboratory conditions i.e. Temperature 23 ± 2 °C, Relative humidity 50 ± 5 %, 12:12 Light: Dark cycle). The animals were fed with standard rat chow and drinking water ad libitum. The rats were acclimatized to the laboratory conditions for a week time. The Institutional Animal Ethics Committee has approved the Experimental protocols and animal use (Resolution No. 60b / 2012 / (i) / a / CPCSEA / IAEC / SVU / MSR – RS dt. 08.07.2012), Sri Venkateswara University, Tirupati, Andhra Pradesh, India.
Chemicals
Cadmium as Cadmium chloride (CdCl2), Zinc as Zinc chloride (ZnCl2), Vitamin C (Ascorbic acid), Vitamin E (α - tocopherol) were obtained from Sigma Chemical Co, Loba Chemicals, India. All the chemicals used in the present study were of the highest purity.
Experimental Design
Rats were divided into ten groups; each contained 6 nos of rats and fed as one of the following diets.
Group 1: Control group
Group 2: CdCl2 dissolved in drinking water @ 10 mg/lit
Group 3: CdCl2 (10 mg / lit of drinking water) + Vitamin C (100 mg / kg BW) through water + Vitamin E (100 mg / kg BW) intraperitonially.
Group 4: CdCl2 (10 mg / lit of drinking water) + Vitamin C (100 mg / kg BW) + Vitamin E (200 mg / kg BW)
Group 5: CdCl2 (10 mg / lit of drinking water) + Vitamin C (100 mg / kg BW) + Vitamin E (300 mg/kg BW)
Group 6: CdCl2 (10 mg / lit of drinking water) + Vitamin C (200 mg / kg BW) + Vitamin E (100 mg/kg BW)
Group 7: CdCl2 (10 mg / lit of drinking water) + Vitamin C (300 mg / kg BW) + Vitamin E (100 mg / kg BW)
Group 8: CdCl2 (10 mg / kg BW of drinking water) + Vitamin C (200 mg / kg BW) + Vitamin E (200 mg / kg BW)
Group 9: CdCl2 (10 mg / kg BW of drinking water) + Vitamin C (200 mg / kg BW) + Vitamin E (300 mg/kg BW)
Group 10: CdCl2 (10 mg / kg BW of drinking water) + Vitamin C (300 mg / kg BW) + Vitamin E (200 mg/kg BW)
The average food consumption by each rat was recorded as 35-60 g of forage / day and drinking water @ 25-40 ml / day.
Soon after the completion of experimental period of 45 days, all the groups of animals were weighed and recorded. Animals were anesthetized and blood samples were collected through cardiac puncture. Animals were sacrificed by cervical dislocation and the tissues like liver and kidney were isolated and kept at 4 °C for further biochemical analysis. Serum samples were separated by using centrifugation at 2000 rpm for 20 minutes and used for biochemical analysis.
The organ weights were presented as Relative Organ was calculated as follows
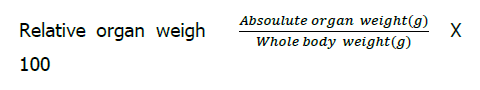
Biochemical parameters selected in the present study were estimated by following methodologies Tissue respiration Statistical Analysis and Data Presentation.9-25
Results obtained were presented as Mean ± SD for comparison. The data were subjected to statistical analysis using SPSS package. The results were statistically analyzed by one way ANOVA. P value < 0.05 was considered as significant. Data of biochemical measurements were further subjected to estimation of percent changes caused by exposure to Cd and co - administration with Vitamin C& E at different combinations.
In the present study ten groups of rats were maintained under different experimental conditions for a period of 45 days. No mortality were observed during the experimental period.
Body Weights and Relative Weights
Initial Body Weights (IBW), Final Body Weights (FBW) and Relative Tissue Weights for Liver and Kidney of male rats subjected to different experimental treatments were obtained and presented in (Table 1). The FBW’s recorded were found to be significantly decreased (p < 0.05) (-46%) in Cd - treated group of rats compared to control group. The FBW’s recorded were found to be ranged in between 241 to 276 g in all the experimental groups of rats treated with different ratios of vitamin C & E.
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| IBW | 220 | 225.19 | 226.11 | 224.11 | 224.79 | 225.18 | 223.72 | 224.45 | 225.11 | 224.77 |
| ±9.48 | ±10.42 | ±10.38 | ±10.18 | ±10.12 | ±10.13 | ±10.14 | ±10.19 | ±10.17 | ±10.34 | |
| FBW | 283.18a | 122.29b | 241.15b,c | 241.03 b,c | 243.04b,c | 276.18 b,c | 242.13b,c | 243.34b,c | 244.73b,c | 244.33b,c |
| ±19.78 | ±5.23 | ±9.32 | ±7.85 | ±8.15 | ±7.79 | ±8.14 | ±8.45 | ±8.74 | ±7.79 | |
| PDC | 28.6 | -45.69 | 6.65 | 7.54 | 8.11 | _22.65 | 8.23 | 8.41 | 8.72 | 8.72 |
| PDE | 97 | 97 | 99 | 126 | 98 | 99 | 100 | 1000 | ||
| LW | 12.14a | 18.71b | 16.12b,c | 15.32b,c | 15.23b,c | 14.62b,c | 15.59b,c | 15.48b,c | 15.68b,c | 15.26b,c |
| ±0.65 | ±0.97 | ±0.75 | ±0.64 | ±0.61 | ±0.58 | ±0.69 | ±0.66 | ±0.71 | ±0.65 | |
| PDC | -54.11 | -32.78 | -26.19 | -25.45 | -20.42 | -28.41 | -27.51 | -29.15 | -25.7 | |
| KW | 3.12a | 4.54b | 4.01b,c | 3.98b,c | 3.89b,c | 3.71b,c | 3.91b,c | 3.97b,c | 4.02b,c | 3.87b,c |
| ±0.29 | ±0.36 | ±0.32 | ±0.25 | ±0.34 | ±0.33 | ±0.26 | ±0.29 | ±0.31 | ±0.27 | |
| PDC | -45.51 | -28.52 | -27.56 | -24.67 | -18.91 | -25.32 | -27.24 | -28.84 | -24.03 | |
| HSI-L | 5.46a | 8.4b | 7.25b,c | 6.89b,c | 6.85b,c | 6.57b,c | 7.01b,c | 6.96b,c | 7.05b,c | 6.86b,c |
| ±0.43 | ±0.71 | ±0.64 | ±0.58 | ±0.57 | ±0.52 | ±0.63 | ±0.54 | ±0.62 | ±0.51 | |
| PDC | 54 | 33 | 26 | 26 | 20 | 28 | 28 | 29 | 26 | |
| HSI-K | 1.40a | 2.04b | 1.80 b,c | 1.79b,c | 1.75b,c | 1.66 b,c | 1.75b,c | 1.78b,c | 1.80b,c | 1.74b,c |
| ±0.09 | ±0.16 | ±0.14 | ±0.13 | ±0.12 | ±0.11 | ±0.13 | ±0.14 | ±0.15 | ±0.13 | |
| PDC | 46 | 29 | 29 | 25 | 19 | 25 | 27 | 29 | 24 |
| Values are expressed as MeanÃÂ?±SD of six individual observations. | Group-1: Control group |
| a,b,c values are not sharing a common superscript letter. (a,b,c) differ significantly at p < 0.05 (DMRT) | Group-2: Cd-treated |
| IBW: Initial Body Weight (g). | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| FBW: Final Body Weight (g). | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| LW: Liver Weight (g). | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| KW: Kidney Weight (g). | Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) |
| HSI-L: Histosomatic Index of Liver. | Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) |
| HSI-K: Histosomatic Index of Kidney. | Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) |
| PDC: Percent Deviation over Control. | Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) |
| PDE: Percent Deviation over Expermental. | Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
The FBW’s recorded was not significant in experimental groups, but it is significant (P < 0.05) when compared to Cd - treated group of rats. Among all the treatments, Cd co - treated with Vitamin C (200 mg) and Vitamin E (200 mg) group of rats were found to be recorded highest FBW and appears to be close to control group of rats and maximum of (+ 126 %) was recorded compared to Cd - treated group of rats and was found to be statistically significant (P < 0.05).
The individual liver weight of rats of different experimental groups showed a significant decrease (P < 0.05) ranges between -20 to -54 % compared to control group of rats. Maximum decrement of -54 % was recorded with Cd - treated group of rats compared to control group, whereas -20 to -32 was recorded with other experimental groups.
The relative weights of kidney were found to be significantly decreased (P < 0.05) in all the experimental group of rats. The present decrease was found to be ranged between -18 to -45% in all the experimental group of rats compared to control group. The Histosomatic Index (HSI) of liver and kidney were found to be significantly decreased (P < 0.05) in all the experimental groups of rats except Cd-treated group of rats which was found to be Not significant (NS).
Respiratory potentials & Glucose metabolism
Basal Metabolic Rate (BMR) at whole animal level and Tissue Respiratory (TR) potentials of both liver and kidney were monitored and presented in (Table 2). The BMR and TR values were found to be significantly (p < 0.05) decreased in all the experimental group of rats compared to control group. The parameters pertaining to glucose oxidation in liver tissue was monitored and presented in (Table 3). The glycogen content was found to be significantly decreased (P < 0.05) and the rate decrease ranges from -17 to -45 %, whereas maximum decrement of -45 % recorded with Cd - treated group of rat liver tissue. Phosphorylase ‘a’ (active) and Aldolase, the key enzymes of glycolysis, responsible for glucose oxidation were found to be significantly (P < 0.05), increased in all the experimental groups of rats compared to control group of rats. Lactate, the end product of anaerobic glycolysis was found to be significantly elevated (P < 0.05) in all the experimental group of rats compared to control group of rats (Table 3). The oxidative enzymes represented by succinate dehydrogenase (SDH) and Cytochrome-C-oxidase activities were found to be significantly inhibited (P < 0.05) in all the experimental group of rats compared to control group. Antioxidant enzymes and products were estimated in liver tissue and presented in (Table 4). Superoxide dismutase (SOD), Catalase (CAT) and Glutathione reductase (GR) were assayed found to be significantly inhibited (P < 0.05), whereas Glutathione Peroxidase (GPx) and Glutathione -S- transferase (GST) enzyme activities were found to be significantly (P < 0.05) elevated in all the experimental groups compared to control group. Antioxidant products represented by Lipid peroxidation (LPO) values were found to be significantly (p < 0.05) elevated, whereas Glutathione reduced content (GSH) was found to be significantly (P < 0.05) decreased in all the experimental group of rats compared to control group. Oxidant and Antioxidant parameters pertaining to kidney of rats after different treatments were estimated and presented in Table 5. Phosphorylase ‘a’ (active) enzyme activity was significantly (P < 0.05) elevated and the range of elevation was found to range between +23 to +40 %, maximum recorded with Cd - treated group of rats. Oxidative damage including SDH & Cytochrome-c-Oxidase and antioxidant enzymes represented by SOD & CAT activities were found to be significantly (P < 0.05) inhibited in all the experimental groups, whereas maximum inhibition was recorded with Cd - treated group of rats. Antioxidant enzymes GPx & GST activities were found to be significantly (P < 0.05) elevated, whereas GR activity levels were significantly (P < 0.05) inhibited in all the experimental group rat kidney tissue. Antioxidant byproducts LPO content was significantly elevated (P < 0.05), whereas GSH levels were found to be significantly (P < 0.05) reduced in all the experimental group of rats. The biochemical parameters pertaining to serum were also estimated and presented in (Table 6). Total Protein, Albumin, Urea, Creatinine, AST, ALT, ALP and LDH were monitored and were found to be altered significantly (p < 0.05) elevated in all the experimental groups of rats extract ALP, which was significantly (P < 0.05) decreased.
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| Basal Metabolic Rate (BMR) | 4.23a | 5.88b | 5.09 b,c | 5.22 b,c | 5.27 b,c | 4.86 b,c | 5.30 b,c | 5.39 b,c | 5.31 b,c | 5.18 b,c |
| ±0.25 | ±0.36 | ±0.31 | ±0.34 | ±0.32 | ±0.26 | ±0.35 | ±0.39 | ±0.36 | ±0.32 | |
| PDC | -39 | -20.33 | -23.4 | -24.58 | -14.89 | -25.29 | -27.42 | -25.53 | -22.45 | |
| Tissue Respiration (TR), Liver | 1145 a | 1667 b | 1468 b,c | 1425 b,c | 1412 b,c | 1341 b,c | 1425 b,c | 1457 b,c | 1425 b,c | 1414 b,c |
| ±22 | ±41 | ±35 | ±32 | ±30 | ±29 | ±33 | ±34 | ±33 | ±30 | |
| PDC | -45.41 | -28.82 | -24.45 | -23.31 | -17.11 | -24.45 | -27.24 | -24.45 | -23.49 | |
| Tissue Respiration (TR), Kidney | 870 a | 1212 b | 1110 b,c | 1111 b,c | 1095 b,c | 735 b,c | 1086 b,c | 1094 b,c | 1093 b,c | 1075 b,c |
| ±13 | ±21 | ±18 | ±20 | ±17 | ±10 | ±15 | ±17 | ±16 | ±14 | |
| PDC | -39.31 | -27.58 | -27.7 | -25.86 | -15.51 | -24.82 | -25.74 | -25.63 | -23.56 |
| BMR: ml of oxygen consumed/ gm weight of animal/hr. | Group-1: Control group |
| TR: µl of oxygen consumed/ gm weight of tissue/hr. | Group-2: Cd-treated |
| Values are expressed as Mean± SD of six individual observations. | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| a,b,c Values are not sharing a common superscript letter (a,b,c) differ significantly at (P<0.05(DMRT)). | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| PDC: Percent Deviation over Control. | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) | |
| Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) | |
| Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) | |
| Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) | |
| Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| Phosphorelase -a | 8.32a | 11.38 b | 10.45 b,c | 10.15 b,c | 10.25 b,c | 9.45 b,c | 10.19 b,c | 10.35 b,c | 10.36 b,c | 10.38 b,c |
| ±0.41 | ±0.71 | ±0.68 | ±0.62 | ±0.63 | ±0.57 | ±0.59 | ±0.64 | ±0.68 | ±0.69 | |
| PDC | 36.77 | 25.6 | 21.99 | 23.19 | 13.58 | 212.47 | 24.39 | 24.51 | 24.75 | |
| Aldolase | 7.45 a | 11.05 b | 9.55 b,c | 9.51 b,c | 9.38 b,c | 8.65 b,c | 9.38 b,c | 9.52 b,c | 9.37 b,c | 9.28 b,c |
| ±0.41 | ±0.65 | ±0.58 | ±0.56 | ±0.52 | ±0.48 | ±0.53 | ±0.54 | ±0.51 | ±0.49 | |
| PDC | 48.32 | 28.18 | 27.65 | 25.9 | 16.1 | 25.9 | 27.78 | 25.77 | 24.56 | |
| Glycogen | 12.39 a | 6.95 b | 8.73 b,c | 9.22 b,c | 9.45 b,c | 10.17 b,c | 9.28 b,c | 8.99 b,c | 9.35 b,c | 9.58 b,c |
| ±0.85 | ±0.61 | ±0.69 | ±0.72 | ±0.74 | ±0.81 | ±0.73 | ±0.69 | ±0.72 | ±0.76 | |
| PDC | -43.9 | -29.53 | -25.58 | -23.72 | -17.91 | -25.1 | -27.44 | -24.53 | -22.67 | |
| Lactate | 1.21 a | 1.75 b | 1.52 b,c | 1.54 b,c | 1.49 b,c | 1.40 b,c | 1.55 b,c | 1.54 b,c | 1.51 b,c | 1.52 b,c |
| ±0.12 | ±0.18 | ±0.14 | ±0.15 | ±0.13 | ±0.13 | ±0.16 | ±0.15 | ±0.13 | ±0.14 | |
| PDC | 44.62 | 24.79 | 27.27 | 23.14 | 15.7 | 28.09 | 27.27 | 24.79 | 24.79 | |
| SDH | 2.84 a | 1.43 b | 2.04 b,c | 1.98 b,c | 2.06 b,c | 2.21 b,c | 2.03 b,c | 2.05 b,c | 2.11 b,c | 2.12 b,c |
| ±0.15 | ±0.08 | ±0.12 | ±0.10 | ±0.12 | ±0.13 | ±0.12 | ±0.10 | ±0.11 | ±0.12 | |
| PDC | -49.64 | -28.16 | -30.28 | -27.46 | -22.18 | -28.52 | -27.81 | -25.7 | -25.35 | |
| Cytocrome-C- Oxidase | 94.78 a | 51.68 b | 66.71 b,c | 65.85 b,c | 67.71 b,c | 74.68 b,c | 67.73 b,c | 70.87 b,c | 69.12 b,c | 70.92 b,c |
| ±3.54 | ±2.13 | ±2.56 | ±2.45 | ±2.58 | ±3.12 | ±2.61 | ±2.89 | ±2.65 | ±2.91 | |
| PDC | -45.47 | -29.62 | -30.52 | -28.56 | -21.2 | -28.53 | -25.22 | -27.07 | -25.17 |
| Values are expressed as Mean± SD of six individual observations. | Group-1: Control group |
| a,b,c Values are not sharing a common superscript letter (a,b,c) differ significantly at (P<0.05(DMRT)). | Group-2: Cd-treated |
| PDC: Percent Deviation over Control. | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| Phosphorelase –a: µmole of Inorganic Phosphate liberated/mg protein/hr | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| Aldolase: µmole of FDP cleaved/mg protein/hr | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| Glycogen: mgs/gram wet weight of tissue. | Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) |
| Lactate: mg/dL | Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) |
| SDH: Succinate dehydrogenase µ moles of formazon formed/mg protein/hr | Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) |
| Cyto-C- O: Cytocrome-C- Oxidase: µ moles of diformazon formed/mg protein/hr | Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) |
| Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| SOD | 72.45 a | 40.83 b | 49.12 b,c | 50.38 b,c | 51.72 b,c | 57.64 b,c | 51.67 b,c | 50.98 b,c | 53.58 b,c | 54.12 b,c |
| ±2.13 | ±1.12 | ±1.32 | ±1.39 | ±1.41 | ±1.46 | ±1.42 | ±1.38 | ±1.39 | ±1.40 | |
| PDC | -43.64 | -32.2 | -30.46 | -28.61 | -20.44 | -28.68 | -29.63 | -26.04 | -25.3 | |
| CAT | 90.45 a | 47.08 b | 62.12 b,c | 64.72 b,c | 63.92 b,c | 69.37 b,c | 65.02 b,c | 65.06 b,c | 65.45 b,c | 66.38 b,c |
| ±2.13 | ±1.10 | ±1.23 | ±1.25 | ±1.22 | ±1.28 | ±1.24 | ±1.24 | ±1.25 | ±1.26 | |
| -47.94 | -31.32 | -28.58 | -29.33 | -23.3 | -28.11 | -28.07 | -27.63 | -26.61 | ||
| GPX | 7.58 a | 11.06 b | 9.71 b,c | 9.81 b,c | 9.74 b,c | 9.28 b,c | 9.63 b,c | 9.72 b,c | 9.68 b,c | 9.78 b,c |
| ±0.42 | ±0.71 | ±0.65 | ±0.66 | ±0.64 | ±0.62 | ±0.63 | ±0.66 | ±0.64 | ±0.65 | |
| PDC | 45.91 | 28.1 | 29.53 | 27.49 | 22.42 | 27.04 | 28.23 | 27.7 | 28.75 | |
| GST | 4.73 a | 6.94 b | 6.05 b,c | 5.89 b,c | 5.93 b,c | 5.76 b,c | 5.98 b,c | 6.03 b,c | 6.10 b,c | 5.95 b,c |
| ±0.25 | ±0.45 | ±0.43 | ±0.37 | ±0.39 | ±0.35 | ±0.39 | ±0.42 | ±0.45 | ±0.39 | |
| PDC | 46.72 | 27.9 | 24.52 | 25.36 | 21.77 | 26.42 | 27.48 | 28.96 | 25.79 | |
| GSH | 6.15 a | 2.74 b | 3.98 b,c | 3.76 b,c | 4.03 b,c | 4.91 b,c | 3.95 b,c | 4.08 b,c | 3.94 b,c | 4.15 b,c |
| ±0.58 | ±0.12 | ±0.28 | ±0.26 | ±0.31 | ±0.34 | ±0.28 | ±0.32 | ±0.29 | ±0.33 | |
| PDC | -55.44 | -35.28 | -38.86 | -34.47 | -20.16 | -35.77 | -33.77 | -35.93 | -32.52 | |
| GR | 6.14 a | 3.45 b | 3.74 b,c | 4.02 b,c | 4.06 b,c | 4.82 b,c | 4.03 b,c | 4.04 b,c | 3.95 b,c | 3.97 b,c |
| ±0.56 | ±0.23 | ±0.26 | ±0.31 | ±0.32 | ±0.39 | ±0.32 | ±0.35 | ±0.27 | ±0.28 | |
| PDC | -43.81 | -38.76 | -34.52 | -33.87 | -21.49 | -34.36 | -34.2 | -35.66 | -35.34 | |
| LPO | 2.34 a | 3.48 b | 3.18 b,c | 3.11 b,c | 3.06 b,c | 2.82 b,c | 3.09 b,c | 3.05 b,c | 3.01 b,c | 2.94 b,c |
| ±0.13 | ±0.26 | ±0.23 | ±0.22 | ±0.19 | ±0.18 | ±0.19 | ±0.17 | ±0.15 | ±0.14 | |
| PDC | 48.71 | 35.89 | 32.9 | 30.76 | 20.51 | 32.05 | 30.34 | 28.63 | 25.64 |
| Values are expressed as Mean± SD of six individual observations. | Group-1: Control group |
| a,b,c Values are not sharing a common superscript letter (a,b,c) differ significantly at (P<0.05(OMRT)). | Group-2: Cd-treated |
| PDC: Percent Deviation over Control. | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| SOD: Superoxide dismutase Units/ g wet weight of tissue/minute. | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| CAT: Catalase activity µ moles of H2O2 degladed/ g wet wt of tissue/ minute. | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| GPX: Glutathione peroxidase nano moles of NADPH oxidized/ mg protein/ minute. | Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) |
| GSH: Glutathione nano moles/ g wet weight of tissue. | Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) |
| GR: Glutathione reductase; µ moles of NADPH oxidized / mg protein / min. | Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) |
| LPO: μ moles of malondialdehyde formed/g. wet wt of tissue. | Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) |
| Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| Phosphorelase -a | 6.77 a | 9.54 b | 8.68 b,c | 8.76 b,c | 8.85 b,c | 8.36 b,c | 8.62 b,c | 8.73 b,c | 8.69 b,c | 8.36 b,c |
| ±0.25 | ±0.49 | ±0.41 | ±0.42 | ±0.43 | ±0.39 | ±0.41 | ±0.42 | ±0.41 | ±0.38 | |
| PDC | 40.91 | 28.21 | 29.39 | 30.72 | 23.48 | 27.32 | 28.95 | 28.36 | 27.47 | |
| SDH | 2.02 a | 1.02 b | 1.25 b,c | 1.30 b,c | 1.32 b,c | 1.56 b,c | 1.34 b,c | 1.37 b,c | 1.36 b,c | 1.42 b,c |
| ±0.12 | ±0.05 | ±0.06 | ±0.07 | ±0.09 | ±0.10 | ±0.09 | ±0.09 | ±0.08 | ±0.07 | |
| PDC | -49.5 | -38.11 | -35.64 | -34.65 | -22.77 | -33.66 | -32.17 | -32.67 | -29.7 | |
| Cyto | 54.45 a | 28.24 b | 34.15 b,c | 35.86 b,c | 35.95 b,c | 41.64 b,c | 35.58 b,c | 35.96 b,c | 33.67 b,c | 38.58 b,c |
| ±2.12 | ±1.23 | ±1.35 | ±1.38 | ±1.39 | ±1.46 | ±1.39 | ±1.40 | ±1.28 | ±1.41 | |
| PDC | -48.13 | -37.28 | -34.14 | -33.97 | -23.52 | -34.65 | -33.95 | -38.16 | -29.14 | |
| SOD | 60.44 a | 33.05 b | 37.25 b,c | 39.65 b,c | 40.61 b,c | 45.55 b,c | 40.55 b,c | 46.57 b,c | 41.95 b,c | 43.65 b,c |
| ±1.77 | ±0.07 | ±0.08 | ±0.09 | ±0.10 | ±0.13 | ±0.09 | ±0.14 | ±0.11 | ±0.12 | |
| PDC | -45.31 | -38.36 | -34.39 | -32.8 | -24.63 | -32.9 | -32.87 | -30.81 | -27.77 | |
| CAT | 73.33 a | 38.75 b | 47.15 b,c | 48.95 b,c | 50.71 b,c | 55.76 b,c | 50.65 b,c | 49.35 b,c | 50.68 b,c | 54.25 b,c |
| ±2.74 | ±1.21 | ±1.33 | ±1.35 | ±1.47 | ±1.55 | ±1.46 | ±1.36 | ±1.48 | ±1.52 | |
| PDC | -47.15 | -35.7 | -33.24 | -30.84 | -23.96 | -30.92 | -32.83 | -30.88 | -26.01 | |
| GPX | 4.77 a | 7.04 b | 6.46 b,c | 6.30 b,c | 6.22 b,c | 5.75 b,c | 6.31 b,c | 6.24 b,c | 6.09 b,c | 6.01 b,c |
| ±0.54 | ±0.82 | ±0.73 | ±0.71 | ±0.69 | ±0.63 | ±0.72 | ±0.70 | ±0.65 | ±0.62 | |
| PDC | 47.79 | 35.42 | 32.07 | 30.39 | 20.54 | 32.28 | 30.81 | 27.67 | 25.99 | |
| GST | 3.49 a | 5.07 b | 4.84 b,c | 4.75 b,c | 4.62 b,c | 4.24 b,c | 4.61 b,c | 4.66 b,c | 4.49 b,c | 4.37 b,c |
| ±0.28 | ±0.42 | ±0.38 | ±0.36 | ±0.34 | ±0.31 | ±0.35 | ±0.37 | ±0.32 | ±0.33 | |
| PDC | 45.27 | 38.68 | 36.1 | 32.37 | 21.48 | 32.36 | 33.52 | 28.65 | 25.21 | |
| GSH | 5.74 a | 3.21 b | 3.75 b,c | 3.89 b,c | 4.01 b,c | 4.55 b,c | 3.77 b,c | 3.87 b,c | 4.10 b,c | 4.16 b,c |
| ±0.57 | ±0.28 | ±0.35 | ±0.36 | ±0.39 | ±0.45 | ±0.62 | ±0.75 | ±0.40 | ±0.41 | |
| PDC | -44.07 | -34.46 | -32.22 | -30.13 | -20.73 | -34.32 | -32.57 | -28.57 | -27.57 | |
| LPO | 1.72 a | 2.51 b | 2.39 b,c | 2.33 b,c | 2.31 b,c | 1.13 b,c | 2.22 b,c | 2.24 b,c | 2.28 b,c | 2.25 b,c |
| ±0.12 | ±0.19 | ±0.17 | ±0.16 | ±0.15 | ±0.08 | ±0.12 | ±0.14 | ±0.13 | ±0.12 | |
| PDC | 45.93 | 38.95 | 35.46 | 34.3 | 23.83 | 29.06 | 30.23 | 32.55 | 30.81 |
| Values are expressed as Mean± SD of six individual observations. | Group-1: Control group |
| a,b,c Values are not sharing a common superscript letter (a,b,c) differ significantly at (P<0.05(OMRT)). | Group-2: Cd-treated |
| PDC: Percent Deviation over Control. | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| Phosphorelase –a: Phosphorelase’a’ (active) activity. | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| SDH: μ moles of formazan formed / mg protein / hour. | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| Cyto-C- O: Cytocrome-C- Oxidase: μ moles of formazan formed / mg protein / hour. | Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) |
| SOD: Superoxide dismutase Units/ g wet weight of tissue/minute. | Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) |
| CAT: Catalase µ moles of H2O2 degladed/ g wet wt of tissue/ minute. | Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) |
| GPX: Glutathione peroxidase nano moles of NADPH oxidized/ mg protein/ minute. | Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) |
| GSH: Glutathione nano moles/ g wet weight of tissue. | Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
| LPO: μ moles of malondialdehyde formed/g. wet wt of tissue. | |
| Parameters | Group-1 | Group-2 | Group-3 | Group-4 | Group-5 | Group-6 | Group-7 | Group-8 | Group-9 | Group-10 |
| TP | 16.24 a | 35.85 b | 29.25 b,c | 28.45 b,c | 27.31 b,c | 23.65 b,c | 26.48 b,c | 26.78 b,c | 26.03 b,c | 25.68 b,c |
| ±1.34 | ±3.12 | ±2.21 | ±2.34 | ±2.25 | ±1.98 | ±1.95 | ±1.98 | ±1.89 | ±1.86 | |
| PDC | 120.75 | 80.11 | 75.18 | 68.16 | 45.62 | 63.05 | 64.9 | 60.28 | 58.12 | |
| AL | 2.54 a | 3.27 b | 3.21 b | 3.19 b | 3.20 b | 3.12 b | 3.21 b,c | 3.25 b,c | 3.19 b,c | 3.22 b,c |
| ±0.24 | ±2.98 | ±2.88 | ±2.82 | ±2.85 | ±2.74 | ±2.92 | ±2.93 | ±2.83 | ±2.87 | |
| PDC | 28.74 | 26.37 | 25.59 | 25.98 | 22.83 | 26.37 | 27.95 | 25.59 | 26.77 | |
| Urea | 32.18 a | 40.72 b | 39.27 b,c | 39.96 b,c | 39.65 b,c | 37.04 b,c | 38.39 b,c | 38.64 b,c | 39.30 b,c | 40.28 b,c |
| ±2.12 | ±3.35 | ±2.89 | ±3.03 | ±3.01 | ±2.65 | ±2.75 | ±2.79 | ±3.25 | ±3.28 | |
| PDC | 26.53 | 22.03 | 24.17 | 23.21 | 15.1 | 19.29 | 20.1 | 22.12 | 25.17 | |
| AST | 56.77 a | 456.15 b | 315.28 b,c | 311.67 b,c | 288.72 b,c | 234.65 b,c | 299.85 b,c | 309.51 b,c | 284.25 b,c | 269.67 b,c |
| ±2.49 | ±18.87 | ±12.39 | ±12.11 | ±10.89 | ±8.94 | ±11.57 | ±11.86 | ±10.75 | ±10.53 | |
| PDC | 719.35 | 455.36 | 449 | 408.57 | 313.33 | 428.18 | 445.19 | 400.7 | 375.02 | |
| ALT | 40.19 a | 166.19 b | 154.01 b,c | 148.43 b,c | 134.75 b,c | 124.65 b,c | 145.75 b,c | 143.55 b,c | 135.75 b,c | 134.35 b,c |
| ±2.42 | ±8.65 | ±7.88 | ±7.12 | ±6.63 | ±6.24 | ±6.98 | ±6.78 | ±6.74 | ±6.68 | |
| PDC | 313.51 | 283.2 | 269.32 | 235.28 | 210.15 | 262.65 | 257.17 | 237.77 | 234.28 | |
| ALP | 110.14 a | 25.65 b | 34.77 b | 39.35 b,c | 45.65 b,c | 68.14 b,c | 47.83 b,c | 53.65 b,c | 58.75 b,c | 56.35 b,c |
| ±6.77 | ±1.38 | ±1.45 | ±1.56 | ±3.15 | ±42.57 | ±3.67 | ±3.36 | ±3.79 | ±3.64 | |
| PDC | -76.71 | -68.43 | -64.27 | -58.55 | -38.13 | -56.57 | -51.28 | -46.65 | -48.83 | |
| LDH | 20.12 a | 45.35 b | 36.44 b,c | 36.22 b,c | 34.67 b,c | 29.84 b,c | 32.48 b,c | 35.72 b,c | 34.31 b,c | 32.81 b,c |
| ±0.45 | ±1.03 | ±0.95 | ±0.92 | ±0.88 | ±0.82 | ±0.86 | ±0.91 | ±0.88 | ±0.89 | |
| PDC | 125.39 | 81.11 | 80.01 | 72.2 | 48.26 | 61.43 | 77.43 | 70.52 | -62.97 |
| Values are expressed as Mean± SD of six individual observations. | Group-1: Control group |
| a,b,c Values are not sharing a common superscript letter (a,b,c) differ significantly at (P < 0.05(OMRT)). | Group-2: Cd-treated |
| PDC: Percent Deviation over Control. | Group-3: Cd (10 mg) + Vit-C (100 mg) + Vit-E (100 mg) |
| TP: Total Protein (mg/ml of Serum) | Group-4: Cd (10 mg) + Vit-C (100 mg) + Vit-E (200 mg) |
| AL: Albumin (mg/ml of Serum) | Group-5: Cd (10 mg) + Vit-C (100 mg) + Vit-E (300 mg) |
| Urea: mg/dl | Group-6: Cd (10 mg) + Vit-C (200 mg) + Vit-E (100 mg) |
| LDH: Lactate Dehydrogenase μ moles of formazan formed / mg protein / hr. | Group-7: Cd (10 mg) + Vit-C (300 mg) + Vit-E (100 mg) |
| AST: Aspartate amino transferase Unit/lit | Group-8: Cd (10 mg) + Vit-C (200 mg) + Vit-E (200 mg) |
| ALT: Alanine amino transferase Unit/lit | Group-9: Cd (10 mg) + Vit-C (300 mg) + Vit-E (300 mg) |
| ALP: Alanine Phosphatase Unit/lit | Group-10: Cd (10 mg) + Vit-C (300 mg) + Vit-E (200 mg) |
In this present study, we presented the toxicological evaluation of Cd and its co-administration with combinations of vitamin C and E. Meanwhile, the remedial effects of vitamin C and E was also analyzed with respect to rehabilitates the altered hepatic and nephrotic functions by the aforesaid Cd, the xenobiotic and antioxidants vitamin C and E, in experimentally designed exposure and duration in wistar rats.
Body Weights and Relative Organ Weights
The measurement of whole body weights and relative organ weights were considered as an important criteria for the evaluation of organ toxicity during toxicological studies, it specifically gives a comprehensive picture about growth rates during toxicity studies. In the present study either decrease or increase in body weights and relative organ weights during Cd - toxicity and Co -administration with different ratios of Vitamin C & E clearly portrays the toxic effects of Cd, the selected xenobiotic in the present study. Both FBW’s and relative organ weights were considerably reduced, manifests the toxic nature of Cd, followed by its action at organ level, thereby reducing the weights significantly. Generally immunization evokes lot of pain, distress and inflammation consequently reducing the animal movement and also appetite, thereby leading to a significant decrease in food uptake i.e. anorexia or food avoidance due to Cd -toxicity. Moreover, the Cd - induced toxicity involves the induction of oxidative stress results in the alterations in the antioxidant status, leading to severe metabolic alterations subsequently leading to heavy weight loss. Reports also available that inflammation causes weight loss ranges between 1 % -20 % due to Cd - toxicity.8-26But in the present study different ratio combinations of Vitamin C and E were found to be more efficient in attenuating the Cd - induced toxicity including metabolic status, increasing food intake, body weights, organ weights to the maximum possible extent.
Respiratory and Antioxidant Status
Generally measurement of oxygen consumption reflects the organismic metabolic status. It is very well established that the respiratory rates are being influenced by both abiotic and biotic factors. The rate of O2 consumption at whole animal level and also at tissue level reflects the metabolic rate, which can be considered as an index of metabolic status of the animal. Therefore, the measurement of O2 consumption can be construed as a bio -detector of pollution of different types including heavy metal pollution, the toxic nature causes stress to the animal. The rate of O2 consumption is taken as a parameter to assess the toxic impact of Cd and will provide useful information on the energy metabolism. In the present study an attempt was made to assess the monitoring of whole animal and tissue respiratory potentials during Cd toxicity and also in other experimental treatments, clearly reveals the metabolic status and also demonstrates that the Cd - toxicity induces hypoxic stress condition in rats and the same was reflected through a decreased O2 consumption at tissue level i.e. liver and kidney tissues. In agreement with Total respiration, the unit metabolism was also showed a significant decrease during Cd-toxicity. This clearly indicates that there was a shift in the metabolic emphasis from aerobiosis to anaerobiosis as a sort of metabolic compensation to overcome the Cd - induced toxic stress. In the present study, an attempt was made to evaluate Cd - toxic effects in rats; furthermore, we are very much interested to know the amelioration potentiality of Vitamin C & E at different proportions in combination along with Cd co - administration in rat tissues. Cadmium has been recognized as one of the most potential toxic environmental pollutant and has been reported to induce oxidative damage at cellular level. Cd -toxicity usually disrupts the prooxidant - antioxidant balance at the cellular level or tissues, thereby induces severe oxidative stress condition. Several authors reported that Cd specifically induced the oxidative damage in several mammalian systems including rats.27,28Mohammad et al. (2018)29 demonstrated that the seriousness of intoxication was governed by the route of administration, dose of exposure and duration of exposure in the manifestation of xenobiotic toxicity at cellular level. Cd mainly accumulates at the cellular level i.e. cytosol (70 %), followed by the nucleus (15 %) and relatively small amounts were also found to be accumulated not only in mitochondria and also endoplasmic reticulum.30 However, due to Cd co -administration with vitamins, metals or chemical supplements facilitates a significant reduction of toxic effects of Cd.27In the present study, both Vitamin C & E combinations successfully ameliorated the Cd - toxicity and restoring to the normalcy in metabolism to the maximum possible extent.
Respiratory Potentials and Glucose Metabolism
Generally, the level of organismic metabolism is determined by measuring the rate of O2 consumption. It is known that the respiratory rates alter under the influence of biotic and abiotic factors. Stress of any type also affects the rate of respiration and metabolism at organism’s level. The rate of O2 consumption of the whole animal as well as tissues reflects the metabolic rate, which can be taken as an index of the metabolic status of the animal. Therefore, measurement of oxygen consumption can be construed as a bio - detector of pollution of different types including heavy metals, the toxic nature cause stress to the animal. O2 consumption at the tissue level indicates differential modulatory responses and the type of metabolism prevalent. These findings also suggest resistance and susceptibility of tissue, which is an important factor for evaluating toxicity. In the present study, an attempt was made to monitor the whole animal and tissue respiratory potentials in the Cd - treated and vitamin C and E Cd - cotreated rat groups to assess the toxicity nature of Cd. The rate of O2 consumption is taken as a parameter to assess the toxic impact of Cd and will provide useful information on the energy metabolism. The results obtained in the present study clearly demonstrates that the Cd - intoxication induces hypoxic stress condition in rats and was reflected through a decreased consumption of O2 at the tissue level i.e. both liver and kidney tissues. It appears that Cd -intoxication requires more energy to mitigate the toxic stress and alters metabolic energy biosynthetic pathways. At tissue level, because of continuous entry of Cd - molecules, tissue metabolism might have undergone a change due to the inhibition of cellular components, concerning metabolism. Respiratory distress is one of the main symptoms of Cd-toxicity and this aspect has been well established in mammalian model system, but it requires in depth study. Both whole animal and tissue respiratory potentials were found to be significantly (P < 0.05) decreased. Moreover, Cd-intoxication it is likely that some histological changes like tissue damage and change (unpublished data) in tissue architecture might be responsible for the observed rate of O2 consumption. Unpublished data in agreement with tissue respiration, the unit metabolism also showed a significant (P < 0.05) decrease under Cd -intoxication. This indicates that there was a shift in the metabolic emphasis from aerobiosis to anaerobiosis as a sort of metabolic conpensation to overcome the Cd - toxicity.
The obtained in the present study also gains support from the earlier reports, wherein Cd - toxicity causes a significant decrease in O2 consumption not only at whole animal and tissue level.26 In the present study, Cd molecules are accumulated in the tissues of rats and appears to change the biochemical profiles of serum, consequent upon its administration. Increased levels of these biomarkers resulted into both hepatic and nephrotic injury. Hepatic and renal damages were elevated globally due to introduction of contaminated factors. The Cd is one of the well-known atmospheric pollutants that are harmful to different tissues including liver and kidney.31 When Cd binds with the metallothionein (MT), which is Cysteine-rich protein, its absorption increases several fold. In the liver, complexes of cysteine metallothionein cause hepatotoxicity and then it goes into the kidney producing nephrotoxicity by a massed in the renal tissue.32 Transportation of Cd into the hepatic system takes place via dual processes concerning the attachment to the cell membrane.32,33 Glycogen was considered as major energy sources and were subjected to oxidation through defined pathways finally yield the biological energy i.e. ATP. Among the tissues of rats generally the liver tissue is considered to be metabolically active in the synthesis and utilization of several metabolites and also specifically involved in the detoxification of both exo and endogenous toxic substances. Cd - toxicity induces rapid utilization of glycogen, by favouring higher rate of glycogenolysis, may be construed as a compensatory phenomenon to counteract the toxic impact of Cd. Phosphorylase enzyme system, which occupies a strategic position in the breakdown of glycogen subsequently glycolytic potential at cellular level. In the present study a significant increase in the Phosphorylase ‘a’ (active) at the tissue level clearly indicates the breakdown of glycogen and its subsequent channeling into glycolysis to counteract the Cd-induced toxic stress. Due to the prevalence of hypoxic condition, i.e. reduced O2 uptake at whole animal and tissue levels, the tissue will explore alternative pathways to overcome the energy requirements under Cd-toxicity.
Aldolase is an important enzyme of the glycolytic pathway and cleaves phosphorylated hexoses to triose phosphates. The enhanced aldolase activity levels trigger the glycolytic potentials and will help in mitigating energy crisis during Cd induced toxicity. The termination of glycolysis under anaerobic conditions in lactic acid production, which is considered to be as an index of existence of physiological stress. In the present study lactate production at tissue level appears to be significant increased, therefore, the tissue lactic acid production and accumulation suggests the tissues capacity to withstand anaerobiosis. Similar kind of situation was also reported in rat tissues under Cd -intoxication32 and also in different animal model systems.29
Mitochondrial Enzyme Systems
The life of every organism depends on its ability to complex compounds and to transform them into simpler molecules for various metabolic purposes and subjected them through oxidation for the production of energy containing biomolecules i.e. ATP and to a large extent is dependent on the availability of molecular O2. The mitochondrial respiratory chain plays an essential role in maintaining energy homeostasis through oxidative phosphorylation, generating energy in the form of ATP, which is essential for life sustainability. The Kreb’s cycle is the main pathway for the oxidation of carbohydrates, lipids and proteins since glucose, fatty acids and amino acids are all converted directly or indirectly into Acetyl-CoA, which is ultimately oxidized into CO2 and H2O, through a series of metabolic reactions. In the present study, an attempt was made to study the oxidative enzymes including SDH and cytochrome-C-oxidase in the rat tissues under Cd-toxicity. Decrease in SDH activity levels as a consequence of Cd-toxicity indicates the impaired mitochondrial oxidation of pyruvate, indicates the depression of oxidative metabolism of the TCA cycle during Cd-induced toxicity. It is quite possible that the decrease in oxidative enzymes must be due to the conditions similar to Asphyxia, because the rate of tissue respiration also diminished under Cd-toxicity in the present study. Since SDH plays a vital role in the oxidative metabolism of the cell, any change in its activity is likely to disturb the harmony and co-ordination of other cellular metabolic processes of the organism. Cytochrome-C-oxidase, which represents Electron Transport System (ETS), an oxygen-dependent process, was significantly inhibited, indicates the impairment in the disturbances in the energy metabolism leading to reduced production of biological energy i.e. ATP.
Antioxidant Enzymes and Products
An antioxidant substance in the cell is present at low concentrations and significantly reduces or prevents oxidation of oxidizable substrate. The antioxidant defense system of the body is one of the multifactorial mechanisms of homeostasis maintenance, and forms the main component network of enzymes, which controls the phenomenon of free radical oxidation initiated by active oxygen species. Mammals have developed highly complex antioxidant systems (both enzymatic and non - enzymatic), which work synergistically and together with each other to protect the cells and organ systems of the body against free radical damage.34 The enzymatic antioxidant defense which includes SOD, Catalase, Peroxiredoxins, Glutathione-S-transferase (GST), coupled to glutathione reductase (GR). SOD is a key enzyme that appears to act as the first line of defense against ROS, the activity levels found to be significantly decreased in the tissues of rats indicates the disturbances in antioxidant defense mechanism, thereby leading to accumulation of ROS molecules. The H2O2 produced by SOD was removed by Catalase or Glutathione peroxidase (GPx). In the present study catalase activity was also considerably inhibited, clearly indicates that conversion of H2O2 to water and oxygen was also diminished. GPx generally catalyse the oxidation of glutathione at direction of hydroperoxide, which may be hydrogen peroxide or another species such as a lipid hydroperoxide. In the present study GPx activity was found to be significantly increased, whereas GSH was significantly decreased, clearly indicates the detoxification of xenobiotic molecules. The antioxidant enzymes such as SOD and CAT were decreased in Cd - intoxicated rats. SOD accelerates the conversion of Superoxide radical (O2-) to hydrogen peroxide, while CAT is an inducible cytosolic enzyme, which serves to protect the biological system against ROS, converting H2O2 to non-toxic oxygen and water. CdCl2 is known to induce LPO, but its effect on antioxidant enzymes is controversial. It has been reported that Cd may induce oxidative damage in a variety of tissues by enhancing peroxidation of membrane lipids due to inhibition of the antioxidant enzymes. LPO is considered to be a primary mechanism for Cd toxicity, despite its inability to directly generate free radicals under physiological conditions. The increase in GPx activity with simultaneous decrease in GR activity in tissues of rats under Cd-intoxication suggests that peroxidase/glutathione reductase system function normally. The changes in tissues, the activity levels of GPx and GR, might be a protective response to peroxides generated under Cd - induced LPO phenomenon. The Glutathione-S-transferase (GST) catalyzes the reactions of toxic substances in conjugation with GSH. The increase in GST activity levels in tissues during Cd - toxicity suggests that GSH must be utilized in the reaction of conjugation. Severe GSH depletion associated with higher level of LPO at the cellular level clearly reveals the occurrence of pathological condition as evidenced through alterations in the histoarchitectural modification at the cell during Cd-toxicity (unpublished data).
Cd toxicity causes structural and functional damage at cell membrane level, significantly increasing permeability resulting in the leakage of hepatic enzymes into blood. Furthermore, the liver damage due to Cd-toxicity was confirmed through the increase in the levels of plasma components, including bilirubin. Therefore, increased activities of ALT and AST in plasma are mainly attributed to the leakage of these enzymes from the liver cytosol into the blood stream. Reports are also available that lysosomal instability caused by Cd - toxicity resulted from leakage of hepatic enzymes including AST, ALT and ALP into blood stream.26 In the present study a significant increase (P < 0.05) in activities of AST, ALT may be attributed to hepatic damage due to Cd - toxicity. The alterations in ALP levels may also be attributed to cholestasis and acute hepatocellular necrosis. Several authors reported that liver enzymes including SGOT, SGPT, ALP activities were found to be significantly elevated in rats subjected to Cd - intoxication, clearly indicates the liver dysfunction. Serum transaminases represented by AST and ALT were significantly elevated during Cd - toxicity, indicating the loss of cellular integrity and the leakage of hepatic membrane. In the present study, the hepatocellular injury was associated with Cd - toxicity (unpublished data). Earlier reports indicates that Cd - toxicity induces significant increase in serum enzymatic activities including ALT, AST, ALP accompanied by a significant decrease in the protein content, and also reflects the destruct effect of Cd on cell membrane, subsequently results in the release of functional enzymes from intracellular locations, which indicates the hepatotoxic nature of Cd.35,32 Reported that ALP, GPT, blood urea were found to be significantly increased after Cd-intoxication. In the present study, serum LDH activity was significantly increased, indicating the hepatocellular necrosis leading to the leakage of enzyme into the blood stream. The liver damage, due to Cd - toxicity will decrease the antioxidant defenses in the tissue. Moreover antioxidant enzymes SOD and CAT were found to be significantly decreased in the present study. These observations indicated that marker enzymatic activity changes followed by the liver’s overall histoarchitecture in response to Cd - toxicity, which could be due to its toxic effects primarily by the generation of ROS causing a significant damage to the various membrane components of the cell.36,37
The prevention of LPO is essential for all aerobic organisms so the organisms are well equipped with antioxidants that capably protect the cells against the adverse effects of various xenobiotics. Antioxidants role in reversing the oxidative stress induced due to xenobiotic intoxication has been of long standing interest to basic scientists and clinicians. In the present study an attempt was made to co-administer with Cd in different proportions of vitamin C & E, efficiently ameliorated the Cd - toxicity, thereby restoring to normal activity in selected tissues to the maximum possible extent. The non-enzymatic antioxidants selected in the investigation are vitamin C & E were considered to be of prime importance. Vitamin C is an important antioxidant and thus works in aqueous environments of the body. Further ascorbic acid can be oxidized in the extracellular environment in the presence of metal ions to dehydroascorbic acid, which is transported into cell through the glucose transportal system. Its primary antioxidant partners are vitamin E as well as working alone with the antioxidant enzymes. Vitamin C in cooperation with vitamin E to generate α-tocopherol from α-tocopherol radicals in membranes and lipoproteins and also raises glutathione levels in the cell, thus playing a vital role in protein thiol group protection against oxidation. Vitamin C is a reducing agent and can reduce and thereby neutralize ROS such as H2O2.31 Vitamin E is a fat-soluble vitamin existing in different forms and are lipid soluble and have pronounced antioxidant properties. They react more rapidly than PUFA with peroxyl radicals and hence act to break the chain reaction of LPO. In addition to its antioxidant role, vitamin E might also have a structural role in stabilizing the membranes. The main function of vitamin E is to protect against LPO, and there is also evidence to suggest that α-tocopherol and ascorbic acid function together in a cyclic-type of process. Due to this close association in performing the antioxidant functions, both vitamin C and E are selected in the present study to monitor the efficiency of amelioration of Cd-induced toxicity in the selected tissues of rats.
In the present study, the combinations of vitamin C & E caused significant alterations pertaining to respiratory potentials, oxidative and antioxidant mechanisms, which promote the amelioration of Cd-induced toxic stress to normalcy or close to normalcy. In the present study, it was observed that Cd-administration showed the highest significant (P < 0.05) elevation / reduction in the levels of both oxidative and antioxidant enzymes in Cd-treated group of rats with respect to control group at the 45th day of experiment. Cd has been testified to be a hepatotoxic and nephrotoxic component. It has been persuaded histopathological alterations in the liver and diminished biomarkers of liver functions in several mammalian systems.3 Both vitamin C & E acts as an antioxidants and plays an important role to defend the hepatic system from oxidative impairment due to Cd - administration. A partial amelioration of this damage by vitamin C & E at different proportions would be attributed to antioxygenic role and vitamin E as a free radical scavenger and an effective inhibitor of autocatalytic process of LPO. Several authors reported that vitamin E is the most important lipophilic antioxidant and exists mainly in the cellular membranes thus helping to maintain the membrane stability.37 Cd - toxicity induces, free radical formation and causes oxidative damage to membrane lipids and lipoproteins, subsequently lead to cellular damage and apoptosis.3,32 Oxidative damage at cell membrane level can further culminate into pathological changes in the histomorphology of xenobiotic exposed tissues. However, vitamin C & E, natural antioxidants, can potently cause inhibition or scavenging of free radicals, thereby reducing the damage effects at tissue level. It is an essential and vitamin C and anti-toxin which potently functions to reduce the harmful effects of toxic agents in biological tissues. Each molecule of Ascorbic acid contains two hydrogen atoms that bear two high-energy electrons, which can be readily donated to reduce oxidation by free radicals, thereby neutralizing or alleviating the harmful effects of tissue toxins.38In the present study though all the combinations of vitamin C and E are effectively reversing the Cd-toxicity, but a combination of vitamin C and E 200 mg each found to be more effective in restoring the Cd-toxicity towards normalcy in metabolic status.
In Conclusion, The present investigation may be concluded that, Cd-toxicity results in a serious depression in respiratory potentials and oxidative metabolism and also induces oxidative damage in selected tissues of rats. The results obtained in the Present study concerning oxidative and antioxidant enzymes clearly demonstrates the effect of Cd-toxicity at cellular and tissue level. With the co-administration of vitamin C & E with Cd, seems to have a protective effect against Cd-induced liver and kidney damage, oxidative stress and apoptosis. Both vitamin C & E selected as antioxidants in the present study showing Cd chelation and antiapoptic activities, which can be considered as key factors associated with the alleviation of both hepato and nephrotoxicity in rats more effectively. Therefore, vitamin C & E were considered as effective agents i.e. 200 mg each combination was more ideal for the prevention of hepatic & renal injury and dysfunctions induced by Cd in rats.
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Googlescholar][Pubmed].
[Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].
[Crossref][Googlescholar][Pubmed].